Your Time has Finished
Loading...
GAMSAT Section III Chemistry – Part 2
Your Score: %
Average Score of All Users:
You performed better than of students
Section Breakdown
| Your Score | Average of all Users | Percentile | |
|---|---|---|---|
| Chemistry - Part 2 |
Chemistry - Part 2
Your score:
Average score:
You performed better than of students
Speed as well as accuracy is important in this section. Work quickly, or you might not finish the paper. There are no penalties for incorrect responses, only marks for correct answers, so you should attempt all questions. Each question is worth one mark.
You must complete the answers within the time limit. Calculators are NOT permitted.
Good Luck!
Maxwell distribution plot
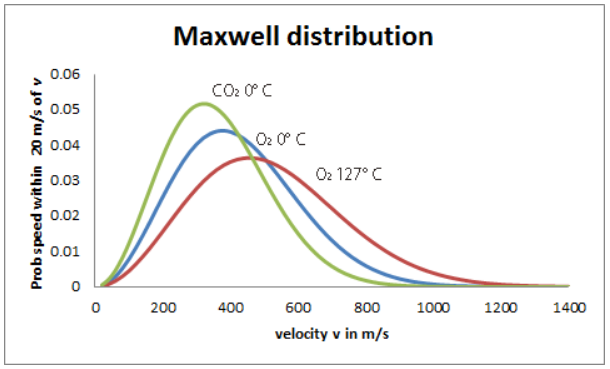
Image reference: https://homepages.abdn.ac.uk/j.s.reid/pages/Maxwell/Legacy/MaxDistrb.html
Explanation
The correct answer is A.
Maxwell distribution plot

Image reference: https://homepages.abdn.ac.uk/j.s.reid/pages/Maxwell/Legacy/MaxDistrb.html
Explanation
The correct answer is C.
Maxwell distribution plot

Image reference: https://homepages.abdn.ac.uk/j.s.reid/pages/Maxwell/Legacy/MaxDistrb.html
Explanation
The correct answer is B.
Maxwell distribution plot

Image reference: https://homepages.abdn.ac.uk/j.s.reid/pages/Maxwell/Legacy/MaxDistrb.html
Explanation
The correct answer is A.
 Charlotte
Medicmind Tutor
Charlotte
Medicmind Tutor
Fri, 12 Aug 2022 08:01:47
I think this answer is wrong?
 Big dick
Medicmind Tutor
Big dick
Medicmind Tutor
Sun, 29 Oct 2023 05:54:25
Y’all crackers be trippin
Raoult’s law
Psolution = Xsolvent x Psolvent
P solution = vapour pressure of solution
X solvent = mole fraction of solvent
P solvent = vapour pressure of solvent
Explanation
The correct answer is B.
Moles of water: 500g/10.00g/mol = 50 moles
Moles of substance A: 300g/150g/mol = 2 moles
50 moles/(50 + 2) moles = 0.96 moles. This is the mole fraction of the solvent. Round to 1
Now enter this into Raoult’s law: 1 x 25 = 25mmHg
Solubility
Explanation
The correct answer is B.
Any compound containing nitrogen or potassium would form a precipitate.
Solubility
Use the graph below to answer the following questions.
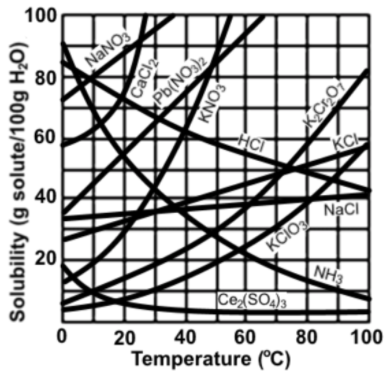
Explanation
The correct answer is C.
From the graph it is clear that this is a highly soluble compound and does not need a high temperature to boost its solubility.
 unistudent
Medicmind Tutor
unistudent
Medicmind Tutor
Tue, 31 Aug 2021 15:33:16
This question is incorrect. The graph clearly shows a temperature dependence of the solubility of NaNO3. Phrases such as "highly dissolvable" can be considered true however B is a much more scientifically correct statement.
 Hyoshin
Medicmind Tutor
Hyoshin
Medicmind Tutor
Wed, 26 Oct 2022 14:46:28
I agree with unistudent, B is objectively more valid based solely on the graph.
Solubility
Use the graph below to answer the following questions.

Explanation
The correct answer is B.
Candidates should read the graph carefully.
Solubility
Use the graph below to answer the following questions.

Explanation
The correct answer is D.
Phase diagrams
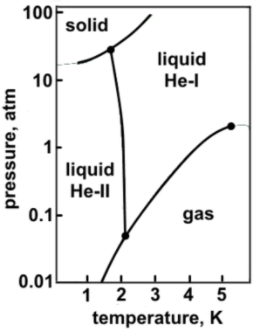
Using the above phase diagram of helium answer the following questions.
Explanation
The correct answer is A.
Phase diagrams

Using the above phase diagram of helium answer the following questions.
Explanation
The correct answer is C.
Phase diagrams

Using the above phase diagram of helium answer the following questions.
Explanation
The correct answer is B.
Phase diagrams

Using the above phase diagram of helium answer the following questions.
Explanation
The correct answer is B.
Candidates should read the graph carefully to answer these questions, no prior knowledge is needed.
Explanation
The correct answer is A.
Explanation
The correct answer is A.
Electronegativity increases moving across and decreases moving down the table.
 unistudent
Medicmind Tutor
unistudent
Medicmind Tutor
Tue, 31 Aug 2021 15:36:06
Assuming this question wasn't written by the actual GAMSAT examiners, the phrase 'electronegativity charge' is incorrect. It is not a charge, but a relative value describing the tendency of a nucleus to attract the bonding pair of electrons in a covalent bond.
 student
Medicmind Tutor
student
Medicmind Tutor
Mon, 28 Feb 2022 12:35:59
B is more electronegative than Be
Explanation
The correct answer is D.
Transition metals have high melting points.
Thermodynamics
Explanation
The correct answer is C.
A substance can absorb heat and use to either increase its temperature or undergo a phase change. As temperature has been increasing over a period of time we can rule out B. We cannot know that the solution is at boiling point so this can also rule out A and D. As temperature increases the velocity of the molecules also increases.
Thermodynamics
Explanation
The correct answer is B.
We cannot know if a phase change or boiling would be reached.
Thermodynamics
Explanation
The correct answer is D.
Specific heat capacity value refers to the amount of heat needed to cause a unit of mass to change its temperature by 1C. Therefore, the greater the specific heat capacity the smaller the temperature change a metal will undergo for a given heat.
Titration
Explanation
The correct answer is A.
Candidates should do 33 x 3 to ascertain that ~100mmol of HCl will be required. This same idea can be used to answer the other questions in this section.
Titration
Explanation
The correct answer is C.
Titration
Explanation
The correct answer is A.
(50/100) x 2.5 = 1.25M
Titration
Explanation
The correct answer is D.
(25/100) x 3 = 75M
Alcohols
Explanation
The correct answer is D.
Ethanol has the shortest chain as is therefore going to have the lowest boiling point.
Alcohols
Explanation
The correct answer is C.
The shorter the alkyl chain the more soluble it is in water.
Alcohols
Explanation
The correct answer is C.
Heptanol is the longest chain and therefore the least soluble.
Alcohols
Explanation
The correct answer is B.
Octanol is the longest chain and will have the highest boiling point.
Alkanes and alkenes
Explanation
The correct answer is B.
Alkanes all end in the suffix -ane.
Alkanes and alkenes
Explanation
The correct answer is C.
All alkenes end in the suffix -ene.
Alkanes and alkenes
Explanation
The correct answer is A.
Alkanes follow the pattern C₂H₂n+₂
Alkanes and alkenes
Explanation
The correct answer is C.
4 Carbons make this butane.
Alkanes and alkenes
Explanation
The correct answer is C.
If double bonds were present they would be unsaturated.
Alkanes and alkenes
Explanation
The correct answer is D.
Alkenes contain double bonds which rules out C. Double bonds are more reactive and easier to break open and add new atoms to.
 Isabella
Medicmind Tutor
Isabella
Medicmind Tutor
Fri, 24 Feb 2023 15:18:22
This is not worded well. Double bonds are stronger than single bonds present in alkanes, and therefore harder to break.
Alkanes and alkenes
Explanation
The correct answer is B.
Universal indicator identifies pH. Limewater is the test for carbon dioxide. Option D is made up.
Aromatic compounds
Explanation
The correct answer is D.
Aromatic compounds
Explanation
The correct answer is A.
Aromatic compounds
Explanation
The correct answer is A.
Aromatic compounds
Explanation
The correct answer is C.
Aromatic compounds
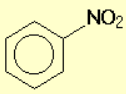
Explanation
The correct answer is C.
Aromatic compounds

Explanation
The correct answer is B.
H is substituted for NO2.
Aromatic compounds
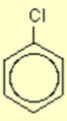
Explanation
The correct answer is D.
 anonymous
Medicmind Tutor
anonymous
Medicmind Tutor
Tue, 08 Feb 2022 19:04:11
why is this wrong and the explanation does not answer this either
Aromatic compounds

Explanation
The correct answer is A.
Addition of a halogen.
Carboxylic acids
Look at the following list of carboxylic acids and use them to answer the questions below.
• Pentanoic acid
• Hexanoic acid
• Heptanoic acid
• Octoanoic acid
• Nonanoic acid
• Decanoic acid
Explanation
The correct answer is D.
The longer the chain the higher the boiling point.
Carboxylic acids
Look at the following list of carboxylic acids and use them to answer the questions below.
• Pentanoic acid
• Hexanoic acid
• Heptanoic acid
• Octoanoic acid
• Nonanoic acid
• Decanoic acid
Explanation
The correct answer is A.
The shorter chains will have a lower boiling point.
Carboxylic acids
Look at the following list of carboxylic acids and use them to answer the questions below.
• Pentanoic acid
• Hexanoic acid
• Heptanoic acid
• Octoanoic acid
• Nonanoic acid
• Decanoic acid
Explanation
The correct answer is B.
The shorter the chain the easier it is to dissolve the acid.
Carboxylic acids
Look at the following list of carboxylic acids and use them to answer the questions below.
• Pentanoic acid
• Hexanoic acid
• Heptanoic acid
• Octoanoic acid
• Nonanoic acid
• Decanoic acid
Explanation
The correct answer is B.
The longer the chain the higher the melting and boiling point. We cannot know based on the table whether adding ketones or mixing it with water would increase melting and boiling points.
Carboxylic acids
Look at the following list of carboxylic acids and use them to answer the questions below.
• Pentanoic acid
• Hexanoic acid
• Heptanoic acid
• Octoanoic acid
• Nonanoic acid
• Decanoic acid
Explanation
The correct answer is A.
The longer the chain the more reactive the carboxylic acid.
Carboxylic acids
Look at the following list of carboxylic acids and use them to answer the questions below.
• Pentanoic acid
• Hexanoic acid
• Heptanoic acid
• Octoanoic acid
• Nonanoic acid
• Decanoic acid
Explanation
The correct answer is B.
The longer the chain the more reactive the carboxylic acid.
Carboxylic acids
Look at the following list of carboxylic acids and use them to answer the questions below.
• Pentanoic acid
• Hexanoic acid
• Heptanoic acid
• Octoanoic acid
• Nonanoic acid
• Decanoic acid
Explanation
The correct answer is D.
The addition of an electronegative substituent increases acidity and thus reactivity. Of all the elements listed Cl is the most electronegative and the most reactive.
Carboxylic acids
Look at the following list of carboxylic acids and use them to answer the questions below.
• Pentanoic acid
• Hexanoic acid
• Heptanoic acid
• Octoanoic acid
• Nonanoic acid
• Decanoic acid
Explanation
The correct answer is C.
The more electronegative and therefore the more acidic a substance the more reactive it is.
Elimination reaction
An elimination reaction is shown below.
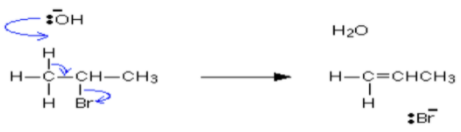
Image reference: https://www.chemguide.co.uk
Explanation
The correct answer is A.
Candidates should use the help of the equation diagram to be able to determine that of the statements only A is correct.
Elimination reaction
An elimination reaction is shown below.

Image reference: https://www.chemguide.co.uk
Explanation
The correct answer is C.
Candidates should be able to see that bromine gains electrons thus ruling out B and D. Candidates should read carefully and identify the inaccuracy in statement A saying carbon-bromide bond.
Elimination reaction
An elimination reaction is shown below.

Image reference: https://www.chemguide.co.uk
Explanation
The correct answer is B.
Candidates can see that the water and hydrocarbon are not charged and that bromide is negative.
Free radicals
Explanation
The correct answer is B.
Free radicals are unstable and highly reactive species, this reactivity stems from an unpaired electron.
Free radicals
Explanation
The correct answer is A.
A free radicals are a naturally occurring phenomemon and are the result of normal cellular respiration, therefore all other answers are false.
Free radicals
Explanation
The correct answer is C.
Pesticide exposure can increase free radicals and this is what candidates should be able to think of when reading unwashed fruit and veg consumption, options A and B are considered part of a healthy lifestyle and candidates cannot know if all medication usage produces free radicals.
Free radicals
Explanation
The correct answer is D.
Candidates should know the hazards cholesterol can pose to health.
 Isabella
Medicmind Tutor
Isabella
Medicmind Tutor
Fri, 24 Feb 2023 15:27:31
Yes excess cholesterol is bad for your health but does it not act in anti-free radical activity? Does it not neutralize them?
Oxidation and reduction
Ascorbic acid protects the body against free radicals, the following equation takes place in the stomach. Use this information to answer the following questions
C₆H₈O₆ + 2H+ +2NO₂¯ -> C₆H₆O₆ + 2H₂0 + 2NO
Explanation
The correct answer is B.
See answer below.
Oxidation is the loss of electrons. Reduction is the gain of electrons. In light of this the reducing agent is the one that becomes oxidised (ascorbic acid) and the oxidising agent is the one that gets reduced.
 Emma
Medicmind Tutor
Emma
Medicmind Tutor
Mon, 11 Mar 2024 21:14:34
Asorbic acid is a reducing agent surely? Asorbic acid has lost H+ and so has been oxidised. And so NO2- is the oxidising agent as it has been reduced?
Oxidation and reduction
Ascorbic acid protects the body against free radicals, the following equation takes place in the stomach. Use this information to answer the following questions
C₆H₈O₆ + 2H+ +2NO₂¯ -> C₆H₆O₆ + 2H₂0 + 2NO
Explanation
The correct answer is A.
Oxidation is the loss of electrons. Reduction is the gain of electrons. In light of this the reducing agent is the one that becomes oxidised (ascorbic acid) and the oxidising agent is the one that gets reduced.
Oxidation and reduction
Ascorbic acid protects the body against free radicals, the following equation takes place in the stomach. Use this information to answer the following questions
C₆H₈O₆ + 2H+ +2NO₂¯ -> C₆H₆O₆ + 2H₂0 + 2NO
Explanation
The correct answer is D.
A hydrogen ion would be H+, devoid of electrons and is thus incorrect.
Oxidation and reduction
MnO4¯ +C2O4¯² -> Mn2⁺² + CO₂²
Explanation
The correct answer is A.
Candidates must balance the equation to determine the coefficient.
Spectroscopy
Spectroscopy is the study of the interaction between matter and light using infrared light.
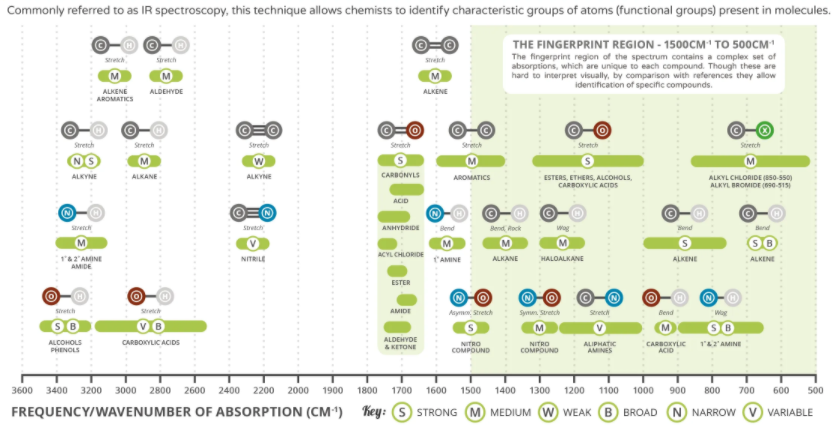
Image reference: https://www.compoundchem.com/2015/02/05/irspectroscopy/
Explanation
The correct answer is A.
Of all the options only A is correct, the other three options contain faults and flaws that make them inaccurate and incorrect.
Spectroscopy
Spectroscopy is the study of the interaction between matter and light using infrared light.

Image reference: https://www.compoundchem.com/2015/02/05/irspectroscopy/
Explanation
The correct answer is C.
Of all the options, only C makes sense considering how spectroscopy works.
Spectroscopy
Spectroscopy is the study of the interaction between matter and light using infrared light.

Image reference: https://www.compoundchem.com/2015/02/05/irspectroscopy/
Explanation
The correct answer is C.
 Isabella
Medicmind Tutor
Isabella
Medicmind Tutor
Wed, 03 Aug 2022 07:39:44
Esters also have a strong wavelength absorption as it is also a carbonyl so all carbonyls should be the answer actually
 Isabella
Medicmind Tutor
Isabella
Medicmind Tutor
Fri, 24 Feb 2023 15:32:45
Why is carbonyls not the answer? Aldehydes have a medium absorption
Spectroscopy
Spectroscopy is the study of the interaction between matter and light using infrared light.

Image reference: https://www.compoundchem.com/2015/02/05/irspectroscopy/
Explanation
The correct answer is B.
Spectroscopy
Spectroscopy is the study of the interaction between matter and light using infrared light.

Image reference: https://www.compoundchem.com/2015/02/05/irspectroscopy/
Explanation
The correct answer is A.
Spectroscopy
Spectroscopy is the study of the interaction between matter and light using infrared light.

Image reference: https://www.compoundchem.com/2015/02/05/irspectroscopy/
Explanation
The correct answer is B.
Spectroscopy
Spectroscopy is the study of the interaction between matter and light using infrared light.

Image reference: https://www.compoundchem.com/2015/02/05/irspectroscopy/
Explanation
The correct answer is D.
Spectroscopy
Spectroscopy is the study of the interaction between matter and light using infrared light.

Image reference: https://www.compoundchem.com/2015/02/05/irspectroscopy/
Explanation
The correct answer is C.
Spectroscopy
Spectroscopy is the study of the interaction between matter and light using infrared light.

Image reference: https://www.compoundchem.com/2015/02/05/irspectroscopy/
Explanation
The correct answer is C.
Spectroscopy
Spectroscopy is the study of the interaction between matter and light using infrared light.

Image reference: https://www.compoundchem.com/2015/02/05/irspectroscopy/
Explanation
The correct answer is A.
Candidates should use the graph to answer all the above questions.
Substitution reactions
Explanation
The correct answer is A.
SN1 reactions are those involving weak nucleophiles. A large, branched electrophile with significant leaving groups will lead to an SN1 reaction, an example is a tertiary alkyl halide. Answer A matches this description.
Substitution reactions
Explanation
The correct answer is B.
SN2 reactions are preferred by reactants that are strong nucleophiles along with a primary electrophile present. The reactants in option B are in keeping with this requirement.
Chemistry - Part 2 Review Screen
Instructions
Below is a summary of your answers. You can review your questions in three (3) different ways.
The buttons in the lower right-hand corner correspond to these choices:
1. Review all of your questions and answers.
2. Review questions that are incomplete.
3. Review questions that are flagged for review. (Click the 'flag' icon to change the flag for review status.)
You may also click on a question number to link directly to its location in the exam.
Chemistry - Part 2 Section
Final Answer Review Screen
Instructions
This review section allows you to view the answers you made and see whether they were correct or not. Each question accessed from this screen has an 'Explain Answer' button in the top left hand side. By clicking on this you will obtain an explanation as to the correct answer.
At the bottom of this screen you can choose to 'Review All' answers, 'Review Incorrect' answers or 'Review Flagged' answers. Alternatively you can go to specific questions by opening up any of the sub-tests below.
Chemistry - Part 2 Section
TI-108
Let's get acquainted ?
What is your name?
Nice to meet you, {{name}}!
What is your preferred e-mail address?
Nice to meet you, {{name}}!
What is your preferred phone number?
What is your preferred phone number?
Just to check, what are you interested in?
When should we call you?
What time works best for you? (UK Time)
How many hours of 1-1 tutoring are you looking for?
My WhatsApp number is...
For our safeguarding policy, please confirm...
For our safeguarding policy, please confirm...
Which online course are you interested in?
What is your query?
SubmitYou can apply for a bursary by clicking this link
https://www.medicmind.co.uk/medic-mind-foundation/Sure, what is your query?
SubmitLoading...
Thank you for your response.
We will aim to get back to you within 12-24 hours.
Lock in a 2 Hour 1-1 Tutoring Lesson Now
If you're ready and keen to get started click the button below to book your first 2 hour 1-1 tutoring lesson with us. Connect with a tutor from a university of your choice in minutes. (Use FAST5 to get 5% Off!)
Buy Now for £70