Your Time has Finished
Loading...
BMAT 2006-S2
Your Score: %
Average Score of All Users:
You performed better than of students
Section Breakdown
| Your Score | Average of all Users | Percentile | |
|---|---|---|---|
| BMAT 2006 S2 |
BMAT 2006 S2
Your score:
Average score:
You performed better than of students
This section is Section 2 of 3.
Speed as well as accuracy is important in this section. Work quickly, or you might not finish the paper. There are no penalties for incorrect responses, only marks for correct answers, so you should attempt all 27 questions. Each question is worth one mark.
You must complete the answers within the time limit. Calculators are NOT permitted.
Good Luck!
Note – if press “End Exam” you can access full worked solutions for all past paper questions
The graph shows changes in the mass of glycogen stored in the liver and skeletal muscles.
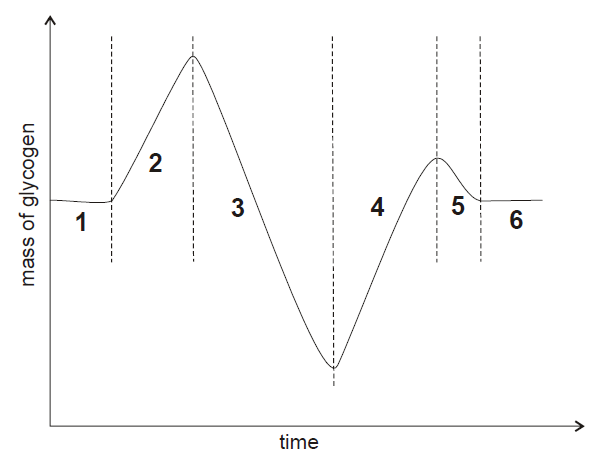
Explanation
The correct answer is E.
Compound T has a melting point of 78 °C and a boiling point of 134 °C. T is soluble in water and its solution does not conduct electricity. T has covalent bonding and has a simple molecular structure.
Explanation
The correct answer is C.
A particular radioisotope X with a half-life of 4 years decays into the stable isotope Y.
At a particular time, a sample contains 32 x 10²º atoms of nuclide X and 4 x 10²º atoms of nuclide Y.
Explanation
The correct answer is F.
The amount of water in urine is controlled by the hormone ADH.
|
|
blood concentration is: | ADH production | nephron action | urine concentration becomes: |
| A | Dilute | falls | less water reabsorbed |
dilute |
|
B |
concentrated | rises | more water reabsorbed | concentrated |
| C | Dilute | rises | less water reabsorbed |
dilute |
|
D |
concentrated | falls | more water reabsorbed | concentrated |
| E | Dilute | falls | more water reabsorbed |
concentrated |
|
F |
concentrated | rises | less water reabsorbed |
dilute |
Explanation
The correct answer is B.
Listed are the electronic configurations for the atoms of different elements.
Explanation
The correct answer is C.
The sides of triangle ABC are as follows:
AB = 3, AC = 2, BC = 4
Use the cosine rule, a 2 = b2 + c 2 – 2bc cos A , to find the cosine of ∠BAC
Explanation
The correct answer is A.
The following statements can be applied to certain types of wave:
1. Their oscillations are longitudinal.
2. They travel at the speed of light in air.
3. They are used in pre-natal scanning.
4. They are used in thermal imaging.
5. They will not travel through a vacuum.
Explanation
The correct answer is A.
A health club monitors the average heart rate of its members when using a cardiovascular workout machine. The lower quartile of the heart rate is 115, the upper quartile is 165.
Explanation
The correct answer is C.
NH₃(g) + HCl(g) ⇋ NH₄Cl(s)
1. Adding a catalyst
2. Adding more ammonia
3. Increasing the pressure
4. Increasing the temperature
Explanation
The correct answer is B.
A gene controls the production of chlorophyll in lettuce plants. The recessive allele of this gene blocks chlorophyll formation which prevents seedling development.
Plants heterozygous for this gene were cross bred and 1000 of their seeds were planted. Only about 750 developed into mature plants.
Explanation
The correct answer is D.
 aria
Medicmind Tutor
aria
Medicmind Tutor
Fri, 06 Oct 2023 13:15:46
exp out of 1000 250 are recessive and hence will die and 500 are hetro 250 are homo for dominant allele now to find percentage of hetro divide 500 by 750 and multiply by 100 to get 67
A force F is applied to the piston X of a brake pedal. This force is transmitted, hydraulically, to piston Y as shown in the diagram.
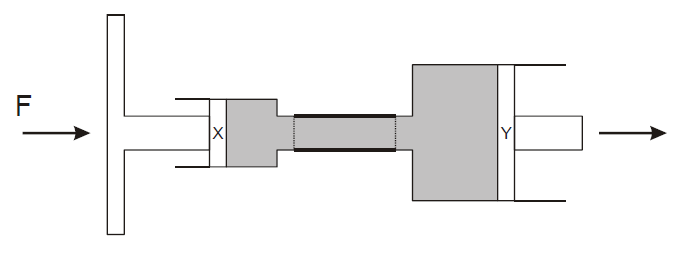
Diameter of piston X = 2 cm.
Diameter of piston Y = 20 cm.
The pressure applied at X is 3 N/cm2
Explanation
The correct answer is C.
 aria
Medicmind Tutor
aria
Medicmind Tutor
Fri, 06 Oct 2023 13:16:18
pascals law pressure will remain same
In statistics Spearman’s rank correlation coefficient is given by the formula:
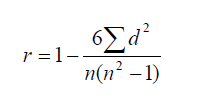
Explanation
The correct answer is E.
At 200 °C, potassium hydrogen carbonate decomposes according to the following equation.
2KHCO₃ → K₂CO₃ +H₂O+ CO₂
Explanation
The correct answer is B.
Find the positive solution of the following simultaneous equations:
4x 2 + y 2 + 10 y = 47
2x – y = 5
Explanation
The correct answer is B.
The diagram shows part of the circulatory system.
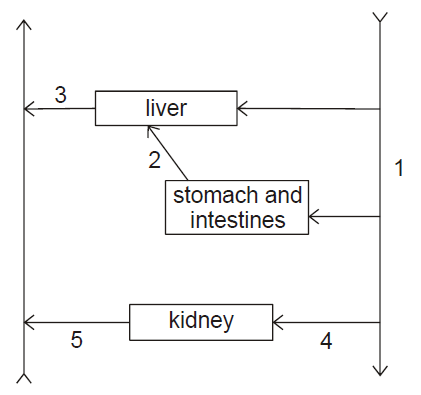
|
|
concentration of substances in the blood | ||
|
highest concentration of glucose |
highest concentration of urea |
lowest concentration of oxygen |
|
| A | 1 | 5 |
4 |
|
B |
5 | 3 | 4 |
| C | 2 | 2 |
3 |
|
D |
1 | 5 | 2 |
|
E |
2 | 3 |
3 |
| F | 5 | 2 |
2 |
Explanation
The correct answer is E.
An object with a mass of 20 kg is lifted by the arrangement shown in the diagram. Air resistance (drag) can be ignored, and the gravitational field strength (acceleration due to gravity) can be taken as 10 N/kg.
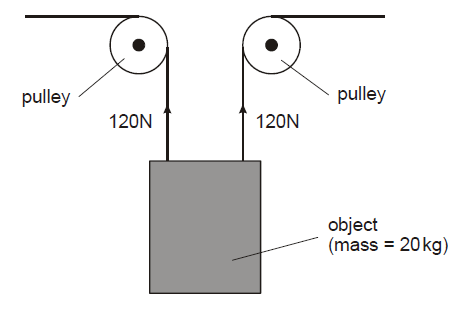
Explanation
The correct answer is B.
The element has a mass number of 40 and an atomic number of 20.
1. The nucleus has a relative mass of 20.
2. It is a noble gas.
3. It would form a negative ion.
4. It is in group 2 of the periodic table.
5. It is a non-metallic element.
Explanation
The correct answer is E.
The table shows some details of the composition of inhaled and exhaled air of a student.
|
Gas |
Inhaled air | Exhaled air |
| Carbon dioxide | 0.03% |
4% |
|
Nitrogen |
78% | 78% |
|
Oxygen |
21% |
16% |
The student is breathing 500 cm³ of air 14 times per minute.
Explanation
The correct answer is C.
Which row of the table shows the response of the iris to reducing levels of light?
|
|
radial muscles | circular muscles | pupil |
| A | contract | relax |
is contracted |
|
B |
relax | contract | is dilated |
| C | contract | relax |
is dilated |
|
D |
relax | contract |
is contracted |
Explanation
The correct answer is C.
Five identical resistors are connected to a cell as in the diagram.
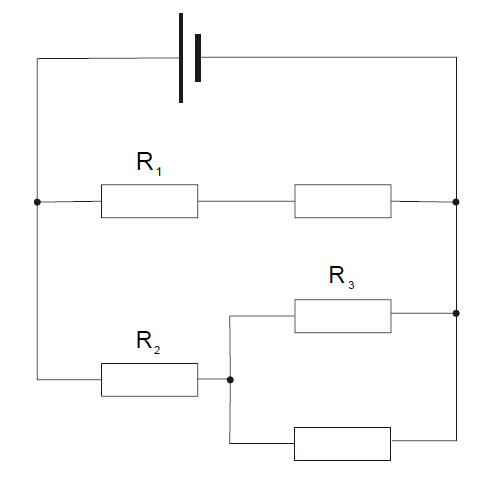
The potential differences across R1 , R 2 and R3 are V1 , V2 and V3 respectively.
Explanation
The correct answer is E.
Evaluate
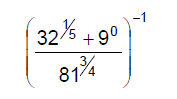
Explanation
The correct answer is B.
The energy profile for a reaction is shown below.
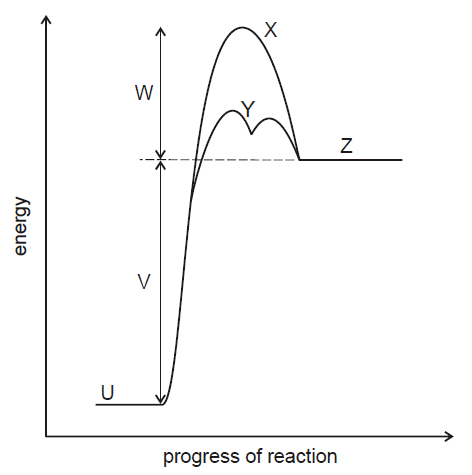
Explanation
The correct answer is D.
The diagram shows the dispersion of white light as it passes from air into glass.
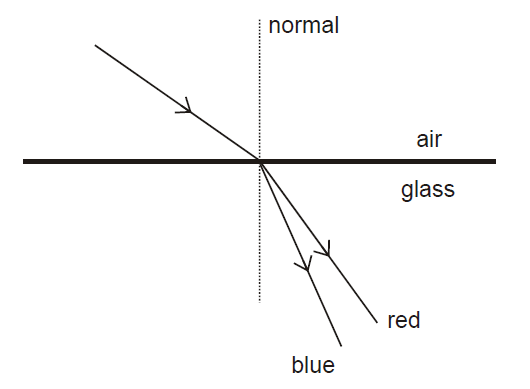
Red light travels at speed c in air, but only 2c/3 in glass. Red light has a wavelength of λ in air.
|
|
frequency of red light in glass | speed of blue light in glass |
| A | 2c/3λ |
< 2c/3 |
|
B |
2c/3λ | > 2c/3 |
| C | c/λ |
< 2c/3 |
|
D |
c/λ | > 2c/3 |
|
E |
3c/2λ |
< 2c/3 |
| F | 3c/2λ |
> 2c/3 |
Explanation
The correct answer is C.
The statements are about respiration.
1. Aerobic respiration releases more energy per unit mass of glucose than anaerobic respiration.
2. Aerobic respiration in muscles causes an oxygen debt to occur.
3. Aerobic respiration forms carbon dioxide as the only waste product.
4. Reduced aerobic respiration will limit the active uptake of mineral ions in the kidney tubules.
5. When there is little oxygen available to muscles only anaerobic respiration occurs.
6. Anaerobic respiration forms lactic acid as the only waste product.
Explanation
The correct answer is E.
In stars four protons, p, are fused together to form helium, 4 He , with a mass of four units, by the following processes.
2p → 2 H + e+
2 2H + p → 3 He
2 3 He → 4 He + 2p
(e+ is a positively charged electron, n is a neutron, 2 H is a hydrogen of mass two and 3 He is helium of mass three.)
Explanation
The correct answer is B.
a is inversely proportional to the square of b.
When a = 9 , b = 4 .
Explanation
The correct answer is A.
A small conducting sphere is held at a fixed distance from a larger sphere which is continually charged by a generator.
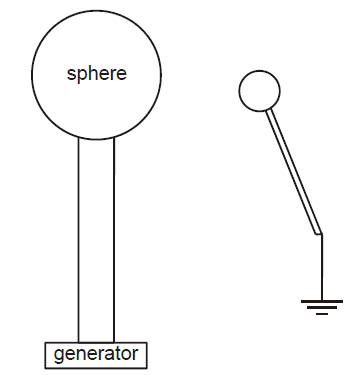
The surface area of the large sphere is 0.04m2 , and it discharges by sparking to the small sphere when the charge density on the large sphere reaches 0.25 C/m².
charge density = charge per unit surface area
Explanation
The correct answer is B.
BMAT 2006 S2 Review Screen
Instructions
Below is a summary of your answers. You can review your questions in three (3) different ways.
The buttons in the lower right-hand corner correspond to these choices:
1. Review all of your questions and answers.
2. Review questions that are incomplete.
3. Review questions that are flagged for review. (Click the 'flag' icon to change the flag for review status.)
You may also click on a question number to link directly to its location in the exam.
BMAT 2006 S2 Section
Final Answer Review Screen
Instructions
This review section allows you to view the answers you made and see whether they were correct or not. Each question accessed from this screen has an 'Explain Answer' button in the top left hand side. By clicking on this you will obtain an explanation as to the correct answer.
At the bottom of this screen you can choose to 'Review All' answers, 'Review Incorrect' answers or 'Review Flagged' answers. Alternatively you can go to specific questions by opening up any of the sub-tests below.
BMAT 2006 S2 Section
TI-108
Let's get acquainted ?
What is your name?
Nice to meet you, {{name}}!
What is your preferred e-mail address?
Nice to meet you, {{name}}!
What is your preferred phone number?
What is your preferred phone number?
Just to check, what are you interested in?
When should we call you?
What time works best for you? (UK Time)
How many hours of 1-1 tutoring are you looking for?
My WhatsApp number is...
For our safeguarding policy, please confirm...
For our safeguarding policy, please confirm...
Which online course are you interested in?
What is your query?
SubmitYou can apply for a bursary by clicking this link
https://www.medicmind.co.uk/medic-mind-foundation/Sure, what is your query?
SubmitLoading...
Thank you for your response.
We will aim to get back to you within 12-24 hours.
Lock in a 2 Hour 1-1 Tutoring Lesson Now
If you're ready and keen to get started click the button below to book your first 2 hour 1-1 tutoring lesson with us. Connect with a tutor from a university of your choice in minutes. (Use FAST5 to get 5% Off!)
Buy Now for £70
Thu, 13 Oct 2022 12:21:01
Why is it E?
Thu, 13 Oct 2022 12:25:30
Why is the answer E?