Your Time has Finished
Loading...
GAMSAT Section III Chemistry – Part 1
Your Score: %
Average Score of All Users:
You performed better than of students
Section Breakdown
| Your Score | Average of all Users | Percentile | |
|---|---|---|---|
| Chemistry - Part 1 |
Chemistry - Part 1
Your score:
Average score:
You performed better than of students
Speed as well as accuracy is important in this section. Work quickly, or you might not finish the paper. There are no penalties for incorrect responses, only marks for correct answers, so you should attempt all questions. Each question is worth one mark.
You must complete the answers within the time limit. Calculators are NOT permitted.
Good Luck!
ELECTROLYSIS AND ELECTROCHEMICAL CELLS
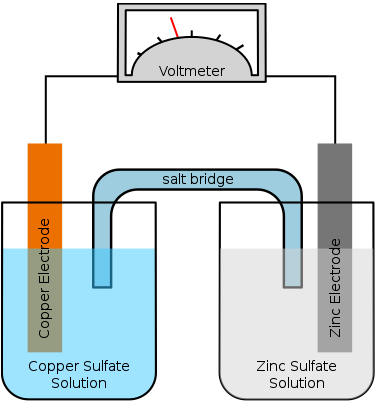
A salt bridge (figure 1) is essential in maintaining electrical neutrality in an electrochemical cell. Without a salt bridge, the two different compartments will accumulate the opposite charges and limit any further reaction.
2Ag⁺(aq) + Cu(s) →2Ag(s) + Cu²⁺(aq)
Explanation
The correct answer is D.
KCl would be a good choice normally because it does not react with any of the chemicals used in the cell, and the anion and cation have similar conductivity, and hence similar migratory speed. In this case, since one of the ions is silver and silver chloride is insoluble, a KCl bridge is not appropriate.
ELECTROLYSIS AND ELECTROCHEMICAL CELLS
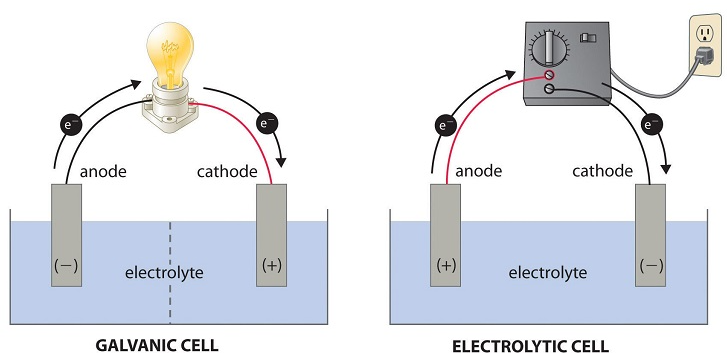
Figure 2 shows a galvanic and electrolytic cell.
Explanation
The correct answer is D.
Galvanic cells have the anode as the negative terminal and cathode as the positive terminal. Here, the signage comes from the anode providing the electrons for the circuit through oxidation.
ELECTROLYSIS AND ELECTROCHEMICAL CELLS
Figure 2 shows a galvanic and electrolytic cell.

Explanation
The correct answer is A.
Ultimately, the anion of the electrolyte will get oxidized, but water would be more easily reduced than most metals. When it gets reduced, hydrogen gas is produced at the anode.
 z
Medicmind Tutor
z
Medicmind Tutor
Tue, 03 Jan 2023 04:46:00
why is c wrong. gases can be produced at the anode; gases be oxygen
ELECTROLYSIS AND ELECTROCHEMICAL CELLS
Zn(s) + 2H⁺(aq) → Zn²⁺(aq) +H₂(g)
Explanation
The correct answer is A.
The equation tells us that the greater the standard electrode potential, the more negative the standard free energy change. So the correct answer is that ∆G° is negative, and E° is positive.
 z
Medicmind Tutor
z
Medicmind Tutor
Tue, 03 Jan 2023 04:48:44
this is wrong. the standard electron potential of zn/zn2+ is -0.76v. it is also negative
 jenny
Medicmind Tutor
jenny
Medicmind Tutor
Mon, 03 Jul 2023 15:05:18
can someone explain this in more detail - why does the equation show that ?
 HAMDA KHAN
Medicmind Tutor
HAMDA KHAN
Medicmind Tutor
Tue, 12 Sep 2023 10:42:19
G=-NFE
 chloe
Medicmind Tutor
chloe
Medicmind Tutor
Wed, 03 Jan 2024 12:11:25
it is asking of the standard electrode potential of the whole reaction (the voltage generated if the reaction were to occur). the E0 of Zn/Zn2+ is -0.76V and the E0 of H2/H+ is 0.00V. the standard electrode potential (of reaction) is calculated by the E0 of the oxidant deducted by the E0 of the reductant. thus, standard electrode potential is 0.00 - (-0.76) = +0.76V.
Amino acids and proteins
Explanation
The correct answer is D.
Acids carry a negative net charge.
Amino acids and proteins
Explanation
The correct answer is C.
For something to have a positive charge it must have a basic R group.
Amino acids and proteins
Explanation
The correct answer is B.
pH is least likely to affect how proteins are formed and amino acids are bonded together, they are more likely to affect the later aspects of protein structure.
 jenny
Medicmind Tutor
jenny
Medicmind Tutor
Mon, 03 Jul 2023 15:07:42
how does pH affect the net charge of a protein ?
 chloe
Medicmind Tutor
chloe
Medicmind Tutor
Wed, 03 Jan 2024 12:05:34
protein is made up of amino acids which have different side chains (called R groups). these R groups could be anything ranging from non polar hydrocarbons to consisting of carboxylic acid groups. eg, in high pH enviro, the acid would deprotonate, which forms COO-, hence the protein's net charge will change.
Amino acids and proteins
Explanation
The correct answer is D.
1st, 2nd and 3rd degree structure all pertain to the specific protein, 4th degree structure is that between multiple peptides.
Atomic structure
Explanation
The correct answer is D.
 Stephanie
Medicmind Tutor
Stephanie
Medicmind Tutor
Thu, 27 Jan 2022 23:06:14
Why is the answer D? 0.7*147 + 0.3*127 = 141
 Sam
Medicmind Tutor
Sam
Medicmind Tutor
Tue, 08 Feb 2022 13:20:33
I agree with Steph. Answer should be 141
 Harriet
Medicmind Tutor
Harriet
Medicmind Tutor
Tue, 09 Aug 2022 13:39:38
Could someone explain how they achieved this answer?
 Bec
Medicmind Tutor
Bec
Medicmind Tutor
Sun, 04 Sep 2022 01:23:00
It should be 14-N and 12-N... 7 is the subscript as its the atomic number... They got the format wrong. Thus its D.
 Ethan
Medicmind Tutor
Ethan
Medicmind Tutor
Sun, 19 Mar 2023 03:58:33
it should be 14*0.7+12*0.3=13.4, the 7 in 147 and 127 were suppose to be the atomic number, they messed up the superscript and subscript
Atomic structure
Explanation
The correct answer is C.
With a half-life of 10 seconds there would be 32 molecules after 10 seconds, 16 after 20, 8 after 30, 4 after 40, 2 after 50, 1 after 60 and 0.5 at 70. Candidates may be inclined to select D but they should note it does not contain the term isotope and also that in regard to half life there is a point where to remaining isotope is present, once less than 1 is reached.
Atomic structure
Explanation
The correct answer is D.
Alpha decay results in the release of He, this will have the greatest impact on nucleus mass.
Atomic structure
Explanation
The correct answer is A.
Candidates can immediately rule out answer b. We need one half life to reach 500kg, three halflives would bring us to 125kg, therefore the half life is 20 years.
Boiling, melting and freezing points
Below is a bar graph showing the melting and boiling points of various elements.
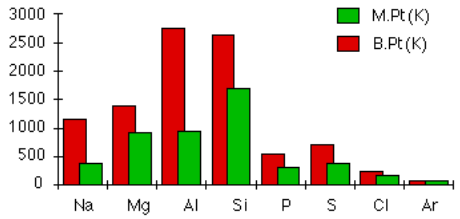
Image reference: https://www.chemguide.co.uk/atoms/structures/period3.html
Explanation
The correct answer is C.
Candidates should be able to reason that considering the information on the right of the bar graph Kelvin is the unit of temperature used.
Boiling, melting and freezing points
Below is a bar graph showing the melting and boiling points of various elements.

Image reference: https://www.chemguide.co.uk/atoms/structures/period3.html
Explanation
The correct answer is A.
Candidates should read the graphs carefully and ensure they use the top of each bar as an indicator for when an element reaches it’s melting and boiling point. Remembering this candidates should then read across from 1000 and determine that answer A is correct as when an element is boiling it is transitioning from a liquid to a gas but this does not mean it is only in a gaseous state.
 Sam
Medicmind Tutor
Sam
Medicmind Tutor
Tue, 08 Feb 2022 13:26:10
But if look at Si we see it doesn't melt until just over 1500K so how can it be liquid at 1000K?
 Isabella
Medicmind Tutor
Isabella
Medicmind Tutor
Tue, 02 Aug 2022 14:52:40
I agree Sam. The answer should be only Na, Mg and Al in my opinion
 Harriet
Medicmind Tutor
Harriet
Medicmind Tutor
Tue, 09 Aug 2022 13:41:41
Could you clarify how Si is liquid at 1000K please?
 Ethan
Medicmind Tutor
Ethan
Medicmind Tutor
Sun, 19 Mar 2023 04:02:26
FYI, Silicon's melting point is 1683 K
Boiling, melting and freezing points
Below is a bar graph showing the melting and boiling points of various elements.

Image reference: https://www.chemguide.co.uk/atoms/structures/period3.html
Explanation
The correct answer is B.
The two bars on the graph are in line with one another, there is not a way to know if the other options are factual based on the information in the graph.
Boiling, melting and freezing points
Below is a bar graph showing the melting and boiling points of various elements.

Image reference: https://www.chemguide.co.uk/atoms/structures/period3.html
Explanation
The correct answer is B.
On the graph at slightly above 1500 the element will have reached its boiling point and is therefore beginning to transition into a liquid state.
 JK
Medicmind Tutor
JK
Medicmind Tutor
Sat, 14 Aug 2021 18:44:36
B.P. --> Liquid state???
 Arthur Jones
Medicmind Tutor
Arthur Jones
Medicmind Tutor
Sat, 04 Sep 2021 09:54:00
The melting point is clearly above 1500
 Alex
Medicmind Tutor
Alex
Medicmind Tutor
Sun, 19 Dec 2021 23:50:13
The melting point means that there will be a phase change from a solid to a liquid. Since the melting point has not been reached yet, it cannot be already in the liquid state. Thus I opted to select 'B' as my answer.
 DC
Medicmind Tutor
DC
Medicmind Tutor
Sun, 26 Dec 2021 10:34:47
Using the same logic as another question, @1500 it isn't necessarily a liquid it's just transitioning. Kind of hard to study when the test can't even be consistant
 Sam
Medicmind Tutor
Sam
Medicmind Tutor
Tue, 08 Feb 2022 13:29:49
B should be the correct answer. If it has not yet reached its melting point then it is a solid . It will not transition to a liquid until the melting point is reached. Only then will it begin to transition
Bonds
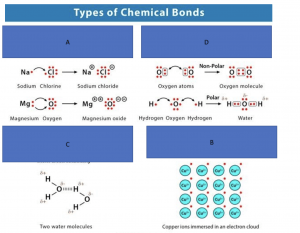
Image reference: https://www.chemistrylearner.com/chemical-bonds
Look at the diagram above and correctly identify the names for each bond type in the following questions
Explanation
The correct answer is A.
Bonds

Image reference: https://www.chemistrylearner.com/chemical-bonds
Look at the diagram above and correctly identify the names for each bond type in the following questions
Explanation
The correct answer is D.
Bonds

Image reference: https://www.chemistrylearner.com/chemical-bonds
Look at the diagram above and correctly identify the names for each bond type in the following questions
Explanation
The correct answer is C.
 Harriet
Medicmind Tutor
Harriet
Medicmind Tutor
Tue, 09 Aug 2022 13:44:18
This diagram is very unclear which is A, B, C or D.
Bonds

Image reference: https://www.chemistrylearner.com/chemical-bonds
Look at the diagram above and correctly identify the names for each bond type in the following questions
Explanation
The correct answer is B.
 Simran
Medicmind Tutor
Simran
Medicmind Tutor
Tue, 03 Aug 2021 09:16:12
The question says identify D but the letter D is nowhere on the diagram, so how are you suppose to answer this?
 Stephanie
Medicmind Tutor
Stephanie
Medicmind Tutor
Fri, 28 Jan 2022 10:17:34
It's extremely hard to see the diagram and the letters A-D are not in order. So this is essentially a trick question.
Explanation
The correct answer is A.
Potassium and chlorine would meet the requirements of an ionic bond.
Explanation
The correct answer is C.
Hydrogen bonds form between hydrogen and electronegative atoms.
Explanation
The correct answer is A.
This is the correct definition for polar and non-polar. Answers C and D are incorrect in information used. Candidates should be able to use the image at the start of the stem to identify what polar and non-polar are.
Explanation
The correct answer is D.
Candidates can use the definition of polar and their knowledge of electrons in each hydrogen and boron to solve this question.
Explanation
The correct answer is D.
A and B are not correct. Candidates may be inclined to choose C but should remember that electrons can be gained or lost to benefit and atom.
Explanation
The correct answer is B.
The energy present holding atoms together in a molecule is potential energy as it has the possibility to be changed into something else.
Bonds
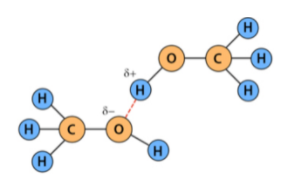
Explanation
The correct answer is C.
There is a hydrogen bond present.
 z
Medicmind Tutor
z
Medicmind Tutor
Tue, 03 Jan 2023 04:51:07
there is also covalent?
 chloe
Medicmind Tutor
chloe
Medicmind Tutor
Wed, 03 Jan 2024 12:25:48
yeah there are also covalent bonds. inaccurate website.
Bonds

Explanation
The correct answer is B.
Potassium has 1 electron in its outer shell and oxygen has 6 therefore 2 potassium and 1 oxygen are required.
 Ethan
Medicmind Tutor
Ethan
Medicmind Tutor
Sun, 19 Mar 2023 04:09:12
please tell me they will give us periodic table for this type of question...
 jenny
Medicmind Tutor
jenny
Medicmind Tutor
Tue, 04 Jul 2023 11:32:53
im not sure but that seems kinda ridiculous to learn numbers for the entire periodic table
Bonds

Explanation
The correct answer is A.
Aluminium has 3 electrons in its outer shell and bromide has 7 therefore you require 3 bromide and 1 aluminium.
Bonds

Explanation
The correct answer is D.
Magnesium has 2 electrons in its outer shell and Nitrogen has 5 electrons in its outer shell therefore 3 magnesium and 2 nitrogen are required to form an ionic bond.
 Charlotte
Medicmind Tutor
Charlotte
Medicmind Tutor
Thu, 11 Aug 2022 10:43:06
Sorry, I still don't understand this question!
 chloe
Medicmind Tutor
chloe
Medicmind Tutor
Wed, 03 Jan 2024 12:34:16
Mg has 2 electrons in its outershell thus wants to lose those 2 electrons to maintain a stable orbit. N has 5 electrons in its outershell thus wants to gain 3 more to maintain a stable orbit (8 electrons in an outershell is considered stable). the 3 Mg will add up to a total of 6 electrons it wants to lose, whereas the 2 N will add up to a total of 6 electrons it wants to gain. hence it is able to form ionic bonds and fulfill each others needs. or u could do the cross and swap method > Mg2+ and N3-, cross and swap and you will get Mg3N2, which means 3Mg and 2N are required.
Bonds

Explanation
The correct answer is C.
Ions that are used to create an ionic compound will cancel each other out and thus the compound will have a neutral charge.
Bonds

Explanation
The correct answer is B.
C and D are not possible, ions are either positive or negative. B should be identified as correct through prior knowledge.
Covalent bonds
Explanation
The correct answer is A.
Candidates should have the prior knowledge to answer this question.
 Mitch
Medicmind Tutor
Mitch
Medicmind Tutor
Fri, 18 Mar 2022 07:13:03
What kind of explanation is this? There's no attempt to explain the reasoning behind why the answer is correct.
Covalent bonds
Explanation
The correct answer is C.
A and B can be ruled out as they do not discuss a difference. Candidates can use prior knowledge to recall that pi bonds form unhybridized bonds therefore D is incorrect.
 Stephanie
Medicmind Tutor
Stephanie
Medicmind Tutor
Fri, 28 Jan 2022 11:41:30
Answer C also do not discuss a difference. Just saying.
Covalent bonds
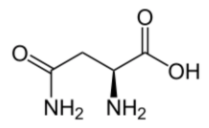
Explanation
The correct answer is C.
The compound is an acid which eliminates answers a and d. There appear to be 4 carbons but one is an amide therefore the answer needs 3 carbons in it which makes C correct.
Covalent bonds
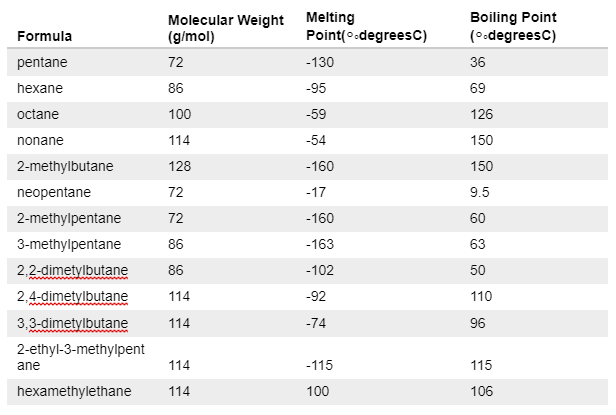
Explanation
The correct answer is D.
Candidates do not require prior knowledge to answer this question. Looking at the table they should be able to identify the inaccuracies of the first 3 statements.
 Stephanie
Medicmind Tutor
Stephanie
Medicmind Tutor
Fri, 28 Jan 2022 11:46:26
Why is B incorrect, it is a true statement.
 Sam
Medicmind Tutor
Sam
Medicmind Tutor
Tue, 08 Feb 2022 13:50:26
B is incorrect because branching also decreases the melting point
 Sam
Medicmind Tutor
Sam
Medicmind Tutor
Tue, 08 Feb 2022 13:52:01
My mistake, Steph is right, B is a true statement
Explanation
The correct answer is C.
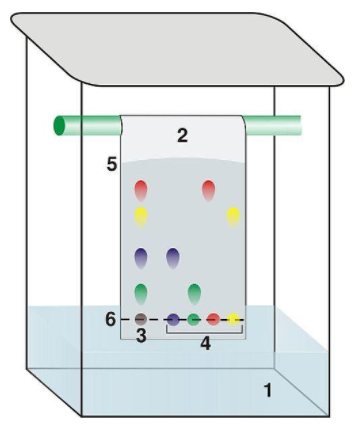
For chromatography to occur a solvent is required, therefore C is correct.
Explanation
The correct answer is B.
Number 4 indicates the individual pigments that make up the mixture indicated by number 3. 1 cannot be known to be a mixture and 6 refers to the pencil line.
Explanation
The correct answer is D.
See explanation below.
In paper chromatography polarity is the factor responsible for separating a mixture into its components. The more polar components of a mixture do not dissolve in a substance, meaning they are the pigments that do not travel as far up the paper.
Explanation
The correct answer is A.
In paper chromatography polarity is the factor responsible for separating a mixture into its components. The more polar components of a mixture do not dissolve in a substance, meaning they are the pigments that do not travel as far up the paper.
Energy diagram question
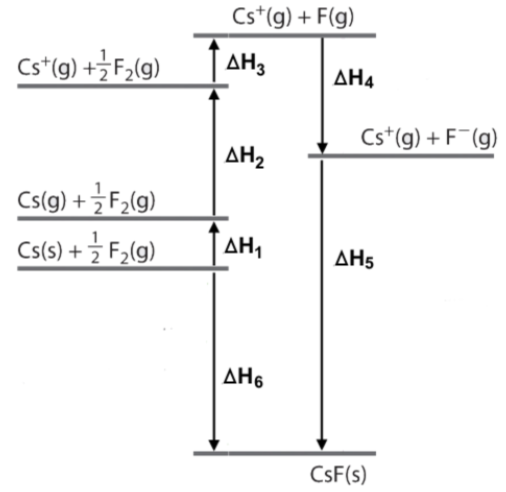
Explanation
The correct answer is B.
CsF is in a solid state coming from gaseous products therefore it is not as the result of heat formation meaning B is incorrect.
 jenny
Medicmind Tutor
jenny
Medicmind Tutor
Tue, 04 Jul 2023 11:37:38
the explanation doesnt make sense - it says that b is incorrect ??
 KiKi
Medicmind Tutor
KiKi
Medicmind Tutor
Fri, 18 Aug 2023 13:50:02
So is B correct or not?
Enzymes.
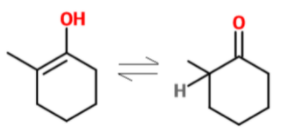
Explanation
The correct answer is D.
 Mitch
Medicmind Tutor
Mitch
Medicmind Tutor
Fri, 18 Mar 2022 07:15:47
Explanation???
 Harriet
Medicmind Tutor
Harriet
Medicmind Tutor
Tue, 09 Aug 2022 14:09:54
Could you explain the reasoning?
Enzymes.
Explanation
The correct answer is A.
 Sam
Medicmind Tutor
Sam
Medicmind Tutor
Wed, 09 Feb 2022 11:41:30
Where is the equation???
 Karthig
Medicmind Tutor
Karthig
Medicmind Tutor
Wed, 24 Aug 2022 15:19:39
It is referring to the equation in the previous question.
Enzymes.
Explanation
The correct answer is B.
As the Km of the reaction is not altered therefore it is not competitive inhibition.
Enzymes.
Explanation
The correct answer is C.
Enzymes lower activation energy making it easier for a reaction to occur.
Hypochlorous acid dissociates in water to hydronium ions and hypochlorite ions.
HOCl + H₂O <-> H3O⁺ + OCl¯
Explanation
The correct answer is B.
Addition of hypochlorite ions shifts the point of equilibria and forms for hypochlorous acid.
Hypochlorous acid dissociates in water to hydronium ions and hypochlorite ions.
HOCl + H₂O <-> H3O⁺ + OCl¯
Explanation
The correct answer is A.
Candidates should use the equation at the top to answer this question.
Hypochlorous acid dissociates in water to hydronium ions and hypochlorite ions.
HOCl + H₂O <-> H3O⁺ + OCl¯
Explanation
The correct answer is C.
This Kb measure indicates the substance to be a strong acid.
 Mitch
Medicmind Tutor
Mitch
Medicmind Tutor
Fri, 18 Mar 2022 07:17:31
Isn't a strong acid the same as a weak base?
 jenny
Medicmind Tutor
jenny
Medicmind Tutor
Tue, 04 Jul 2023 11:39:48
why would b be incorrect - Kb is the base dissociation constant
 chloe
Medicmind Tutor
chloe
Medicmind Tutor
Wed, 03 Jan 2024 12:42:22
a strong acid has a weak conjugate base. given that HOCl is a strong acid, its conjugate base, OCl-, will be a weak base.
Hypochlorous acid dissociates in water to hydronium ions and hypochlorite ions.
HOCl + H₂O <-> H3O⁺ + OCl¯
Explanation
The correct answer is C.
pH is the inverse of the log therefore it is -4.
 Isabella
Medicmind Tutor
Isabella
Medicmind Tutor
Tue, 02 Aug 2022 14:59:20
Isn't the pH scale from 0 to 14? So how can you have a negative pH value?
 Jocelyn
Medicmind Tutor
Jocelyn
Medicmind Tutor
Fri, 19 Aug 2022 08:30:29
I agree with Isabella, can you please explain why is the answer -4?
 chloe
Medicmind Tutor
chloe
Medicmind Tutor
Wed, 03 Jan 2024 12:45:21
a negative pH is possible as long as there is a high H+ concentration. pH of the solution is calculated by -log(100000) = -4
Explanation
The correct answer is C.
As ammonia is a weak base the solution remains slightly acid.
Explanation
The correct answer is B.
Adding more of the weak base will enable a neutral pH to be reached.
Explanation
The correct answer is D.
Strong bases ionise almost irreversibly therefore their products are weak conjugate acids.
 Mitch
Medicmind Tutor
Mitch
Medicmind Tutor
Fri, 18 Mar 2022 07:19:01
Strong acids also have weak conjugate bases????
 KiKi
Medicmind Tutor
KiKi
Medicmind Tutor
Fri, 18 Aug 2023 14:02:07
Agree with Mitch, the question doesn't specify which conjugate to choose. You can immediately rule out A and C, however D is not technically the only correct answer.
Rate law
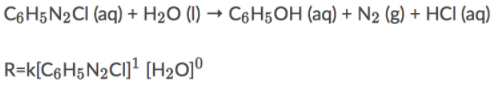
Explanation
The correct answer is B.
The overall order for a reaction is the sum of the individual reaction orders.
Rate law
R=k[C₆H₅N₂Cl]² [H₂O]¹
Explanation
The correct answer is D.
The overall order for a reaction is the sum of the individual reaction orders.
Rate law
Explanation
The correct answer is C.
Candidates should know what a catalyst is and be able to identify that answers B and D are not correct options. Candidates should be able to reason that the slowest aspect of a reaction will dictate its overall rate not the fastest aspect of it.
Rate law
Explanation
The correct answer is A.
Candidates should be able to identify that of the statements either A or B are going to be correct and that a temperature increase will accelerate the rate of a reaction.
 Stephanie
Medicmind Tutor
Stephanie
Medicmind Tutor
Fri, 28 Jan 2022 23:06:16
How can you have a multiple choice question that only allows one answer and have more than one be correct?!
 Ethan
Medicmind Tutor
Ethan
Medicmind Tutor
Sun, 19 Mar 2023 04:18:22
the explanation is obviously wrong, T has proportional relation with reaction rate, so B is wrong. The only scenario where decrease T would "positively" affects a reaction is during an endothermic reaction, but what been affected in that case is equilibrium constant but not reaction rate.
Hybridisation
Explanation
The correct answer is A.
Consider the number of electrons present for boron and how the s and p shells fill.
Hybridisation
Explanation
The correct answer is B.
Main group elements has one s and 3 p orbitals in their valence shell that can be involved in bonding. The shape of the molecule is what identifies how these orbitals mix together, in this case it is a trigonal planar shape. This means that there will be an s and 2 p orbitals used.
 Ethan
Medicmind Tutor
Ethan
Medicmind Tutor
Sun, 19 Mar 2023 04:20:50
one simple trick to solve this kind of question is by counting number of lone pair electron and sigma bond. In this case 0 lone pair, 3 single bond, add them up and match with the hybridisation with same power sum (S1P2)
Hybridisation
Explanation
The correct answer is A.
Linear shapes have central atoms with 2 sets of bonds and no lone pairs therefore there are two hybrid orbitals.
Hybridisation
Explanation
The correct answer is C.
Consider the shape which requires an s orbital and 3 p orbitals to form it.
Lewis dot structures
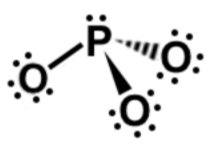
Explanation
The correct answer is D.
 Mitch
Medicmind Tutor
Mitch
Medicmind Tutor
Fri, 18 Mar 2022 07:21:09
Explanation???
 Jocelyn
Medicmind Tutor
Jocelyn
Medicmind Tutor
Fri, 19 Aug 2022 08:42:59
Is this answer accurate?
 Will
Medicmind Tutor
Will
Medicmind Tutor
Mon, 25 Mar 2024 15:24:34
this answer is correct
Lewis dot structures
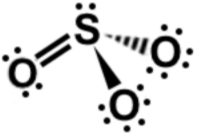
Explanation
The correct answer is A.
 Isabella
Medicmind Tutor
Isabella
Medicmind Tutor
Tue, 02 Aug 2022 15:01:56
What is the explanation?
 Ethan
Medicmind Tutor
Ethan
Medicmind Tutor
Sun, 19 Mar 2023 04:25:40
A simple formula to solve this question is Number electron in outer shell of presents form - Number electron presents in diagram = charge. In this case 24 - 26 = -2 (any line counts as 2 electron in diagram)
Lewis dot structures
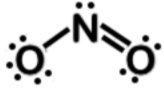
Explanation
The correct answer is A.
 Isabella
Medicmind Tutor
Isabella
Medicmind Tutor
Tue, 02 Aug 2022 15:02:09
What is the explanation?
Lewis dot structures
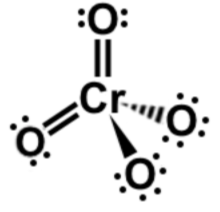
Explanation
The correct answer is B.
 Isabella
Medicmind Tutor
Isabella
Medicmind Tutor
Tue, 02 Aug 2022 15:02:22
What is the explanation?
Liquids
Explanation
The correct answer is D.
The more dense a liquid the longer it would take an object to sink through it.
 Steph
Medicmind Tutor
Steph
Medicmind Tutor
Sat, 09 Sep 2023 23:04:23
i thought buoyant force was determined by volume of liquid and density of object??
Liquids
Explanation
The correct answer is A.
The third law states there are equal and opposite forces acting against each other to keep an object in equilibrium therefore the scale will register a higher mass.
 Ethan
Medicmind Tutor
Ethan
Medicmind Tutor
Sun, 19 Mar 2023 04:28:31
The explanation makes no sense, I consider "suspend" means to hang the block and lowered to water, wouldnt the buoyant force cancels the gravitational force?
 KiKi
Medicmind Tutor
KiKi
Medicmind Tutor
Fri, 18 Aug 2023 14:21:50
I read the question the same way Ethan did, can someone explain?
Liquids
Explanation
The correct answer is B.
Pascal’s law states that pressure is transmitted undiminished to every portion of fluid and wall of a container therefore the forces of the pistons must be equal.
 Stephanie
Medicmind Tutor
Stephanie
Medicmind Tutor
Fri, 28 Jan 2022 23:26:27
If, as you say, "Pascal’s law states that pressure is transmitted undiminished to every portion of fluid and wall of a container therefore the forces of the pistons must be equal.", then how is B correct when it talks about volume, while C, which is what I chose, talks about force, which in liquids, is pressure?!
 Will
Medicmind Tutor
Will
Medicmind Tutor
Mon, 25 Mar 2024 15:28:58
Use process of elimination here, A and C are incorrect and D doesn't refer to pascals law
Liquids
A 30kg block is dropped into acetic acid which has a specific gravity of 1.06. Once in the acid the block weighs 240N. Use this information to answer the following questions.
Explanation
The correct answer is D.
 Lois
Medicmind Tutor
Lois
Medicmind Tutor
Mon, 22 Aug 2022 19:42:38
how do we do this calculation?
 Mig
Medicmind Tutor
Mig
Medicmind Tutor
Fri, 03 Mar 2023 15:25:44
They basically just rounded grvaity (9.81) to 10, force/mass = gravity and just rearrange
 Ethan
Medicmind Tutor
Ethan
Medicmind Tutor
Sun, 19 Mar 2023 04:30:42
Why would they put a physics question in chemistry section??
Liquids
A 30kg block is dropped into acetic acid which has a specific gravity of 1.06. Once in the acid the block weighs 240N. Use this information to answer the following questions.
Explanation
The correct answer is C.
Liquids
A 30kg block is dropped into acetic acid which has a specific gravity of 1.06. Once in the acid the block weighs 240N. Use this information to answer the following questions.
Explanation
The correct answer is C.
 Mitch
Medicmind Tutor
Mitch
Medicmind Tutor
Fri, 18 Mar 2022 07:24:24
Explanation??
 jenny
Medicmind Tutor
jenny
Medicmind Tutor
Tue, 04 Jul 2023 11:47:45
can anyone explain this ?
 KiKi
Medicmind Tutor
KiKi
Medicmind Tutor
Fri, 18 Aug 2023 14:27:31
How I explained it to myself : The box weighed 300N, once in the acid it weighed 240N. So 300N - 240N = 60N. Making the answer C.
Liquids
A 30kg block is dropped into acetic acid which has a specific gravity of 1.06. Once in the acid the block weighs 240N. Use this information to answer the following questions.
Explanation
The correct answer is A.
Liquids
A 30kg block is dropped into acetic acid which has a specific gravity of 1.06. Once in the acid the block weighs 240N. Use this information to answer the following questions.
Explanation
The correct answer is D.
 jenny
Medicmind Tutor
jenny
Medicmind Tutor
Tue, 04 Jul 2023 11:48:17
explanation?
Liquids
A 30kg block is dropped into acetic acid which has a specific gravity of 1.06. Once in the acid the block weighs 240N. Use this information to answer the following questions.
Explanation
The correct answer is A.
Chemistry - Part 1 Review Screen
Instructions
Below is a summary of your answers. You can review your questions in three (3) different ways.
The buttons in the lower right-hand corner correspond to these choices:
1. Review all of your questions and answers.
2. Review questions that are incomplete.
3. Review questions that are flagged for review. (Click the 'flag' icon to change the flag for review status.)
You may also click on a question number to link directly to its location in the exam.
Chemistry - Part 1 Section
Final Answer Review Screen
Instructions
This review section allows you to view the answers you made and see whether they were correct or not. Each question accessed from this screen has an 'Explain Answer' button in the top left hand side. By clicking on this you will obtain an explanation as to the correct answer.
At the bottom of this screen you can choose to 'Review All' answers, 'Review Incorrect' answers or 'Review Flagged' answers. Alternatively you can go to specific questions by opening up any of the sub-tests below.
Chemistry - Part 1 Section
TI-108
Let's get acquainted ?
What is your name?
Nice to meet you, {{name}}!
What is your preferred e-mail address?
Nice to meet you, {{name}}!
What is your preferred phone number?
What is your preferred phone number?
Just to check, what are you interested in?
When should we call you?
What time works best for you? (UK Time)
How many hours of 1-1 tutoring are you looking for?
My WhatsApp number is...
For our safeguarding policy, please confirm...
For our safeguarding policy, please confirm...
Which online course are you interested in?
What is your query?
SubmitYou can apply for a bursary by clicking this link
https://www.medicmind.co.uk/medic-mind-foundation/Sure, what is your query?
SubmitLoading...
Thank you for your response.
We will aim to get back to you within 12-24 hours.
Lock in a 2 Hour 1-1 Tutoring Lesson Now
If you're ready and keen to get started click the button below to book your first 2 hour 1-1 tutoring lesson with us. Connect with a tutor from a university of your choice in minutes. (Use FAST5 to get 5% Off!)
Buy Now for £70