Loading...

COVID-19 Vaccines: Everything You Need To Know For Medical Interviews
Note: this article was written in January 2022. While the information described here is still relevant and valid, you should also research more recent developments too.
Preparing for medical school interviews can be as nerve-wracking as it is exciting. On one hand, you’re standing on the threshold to one of the greatest experiences of your life – medical school – but on the other, the knowledge expected of you is vast and the competition tough. One of our most effective preparation tips is to focus on the major headlines within the healthcare realm. Keeping this in mind, we’re bringing you the ultimate guide to this year’s most touted feat – COVID-19 vaccines.
A breakdown of COVID-19 vaccines
As of January 2022, there are 9 vaccines that have been approved by various national regulatory authorities around the world. Of these, the Pfizer-BioNTech, Oxford-AstraZeneca and Moderna vaccines have been approved by the UK following thorough assessments by the Medicines and Healthcare products Regulatory Agency (MHRA).
For medical school interviews in the UK, dialogue will most likely be centered around the aforementioned trio. Consequently, the remainder of this guide will walk you through the information you need about these vaccines. However, there is no harm in briefly reading about the various other vaccines being trialled around the world.
Pfizer-BioNTech
The Pfizer-BioNTech vaccine was the first vaccine to be approved in the UK. The vaccine was developed by a German firm BioNTech and US drug giants, Pfizer.
The vaccine is an mRNA vaccine, which is made up of a mutated form of a spike protein found on the virus. The mRNA technology is relatively new for vaccines, and this has led to some of the concerns around the safety of the Pfizer vaccine, much of which is driven by social media and anti-vaccination campaigns.
The Pfizer vaccine has been shown to be 95% effective in clinical trials. In the trials, the patients received two doses, given 21 days apart. The timing of the doses is a hot topic in the news, as the UK is the only country to have delayed the second dose of the vaccine. The UK argues that the first dose of the vaccine provides significant protection, so it has decided to maximise the number of people vaccinated with the first dose.
The UK strategy comes at a risk, as although more people have been vaccinated with the first dose, there are questions over how effective the vaccine will be with a 3 month delay between dose 1 and dose 2, rather than a 21 day gap. This move has been highly debated with Pfizer, BioNTech and WHO disagreeing with the government’s decision. It is worth considering the scientific and ethical advantages and disadvantages of this move in preparation for your interview.
Kickstart your Interview Prep
Oxford-AstraZeneca
Oxford-AstraZeneca was the second vaccine to be approved in the UK. Locally developed, this vaccine is a recombinant adenoviral vector vaccine. Breaking this term down will help reveal a lot about the its properties;
- Recombinant refers to the use of small amounts of genetic material from the SARS-Cov-2 virus to modify the weakened adenovirus used in the vaccine. This cannot reproduce a COVID-19 illness in recipients.
- Adenoviral vector refers to the adenovirus obtained from Chimpanzees and used in a weakened form within the vaccine.
Preliminary results from the phase III trial were published in early December, however, Reuters uncovered discrepancies with dosing in some test groups. Due to the dosing discrepancies, the efficacy of the vaccine could not be pinpointed thereby lending the currently accepted range of 62%-90%. This vaccine is also given in 2 doses, between 4 and 12 weeks apart. However, it is worth noting that adenovirus-based vaccines may require booster shots – a concept that is yet to be thought necessary for the other two vaccines. This has not yet been deemed necessary for the Oxford-AstraZeneca vaccine and is only a theoretical concern as of now.
Moderna
Moderna, developed by an American biotechnology firm of the same name was the third vaccine to be approved in the UK in early January. Using similar technology to the Pfizer vaccine, this is also a mRNA vaccine based on the building of a spike protein similar to that of the SARS-Cov-2 virus. In November, preliminary results from the phase III trial showed a 94.1% efficacy in preventing COVID-19 illnesses. This, like it’s counterparts, is given in two doses, 28 days apart.
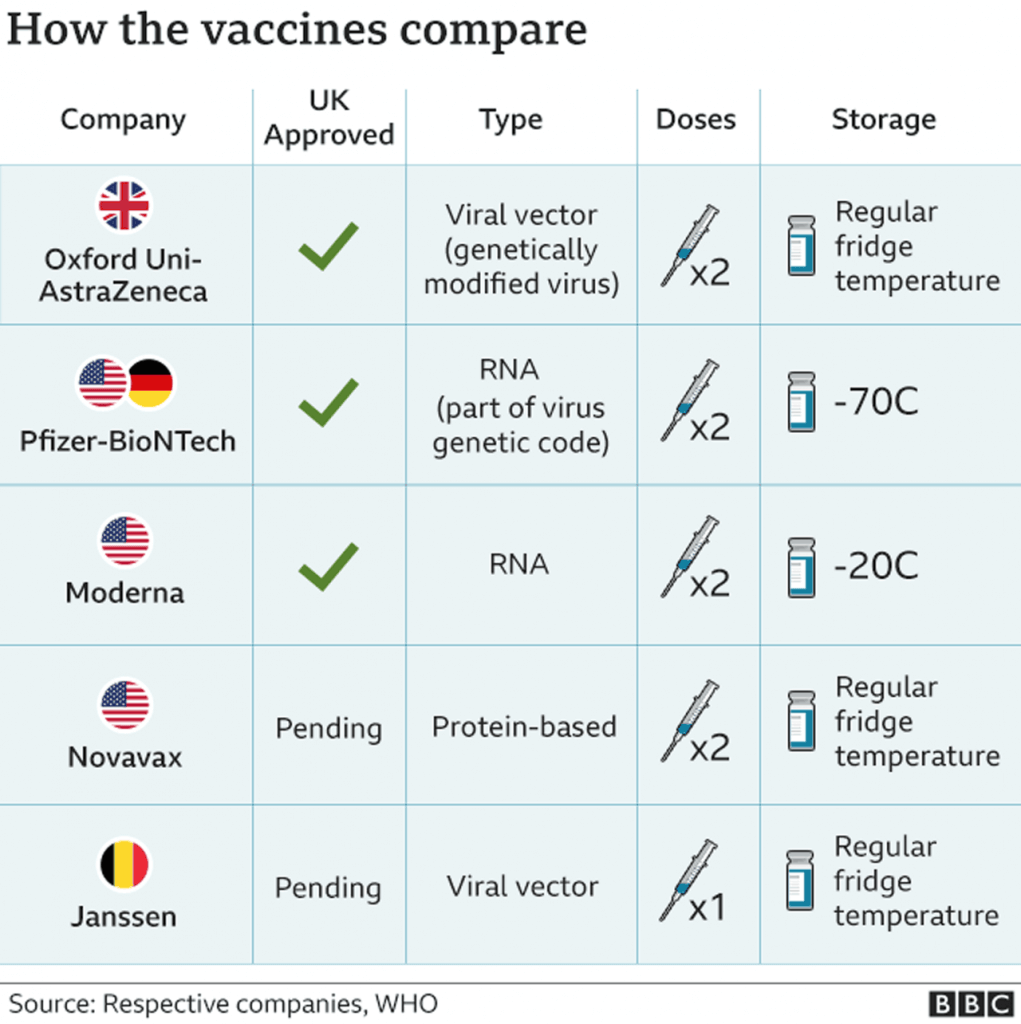
There is a wealth of information available regarding all 3 vaccines. The table below best encapsulates the biggest differences between them. It is worth considering these when reflecting on the rollout and uptake of the vaccines by the general public. Other considerations include cost, logistics and the recent EU row with AstraZeneca that may impact the delivery of Pfizer-BioNTech vaccines to the UK.
| Pfizer-BioNTech | Oxford-AstraZeneca | Moderna | |
| Technology | mRNA-based | Adenovirus-based | mRNA-based |
| Efficacy | 95% | 62%-90% | 94.1% |
| Storage | -70C | 2C to 8C | -25C to -15C |
| Vaccine Rollout | 40 million doses have been purchased but the second dose has been delayed beyond the 21 days indicated by Pfizer and BioNTech | 100 million doses have been purchased with the second dose delayed; however, this has been approved by Oxford | 7 million doses have been purchased making this the least popular option available to the UK population |
All 3 vaccines have a similar profile of mild side effects lasting up to a week following the jab. These include soreness at the site of the injection, fatigue, headaches and/or nausea. The vaccines are currently contraindicated in those with a previous history of anaphylactic shock due to the risk of severe allergic reactions; it is vital to note that this is extremely rare.
How mRNA vaccines work
Vaccines commonly work by injecting an antigen, which is a weakened or inactive part of the pathogen, which triggers an immune response within the body. An mRNA vaccine contains mRNA made in the lab, which contains the genetic code for the person’s cells to produce the vaccine antigens and produce an immune response. This means that instead of injecting the antigen into the human body but instead the instructions on how to produce the antigens are injected. Our cells then present antigens on their surface, providing immunity once exposed to the pathogen. The main benefits of an mRNA vaccine are that it can be developed rapidly and the pathogen isn’t needed to make the vaccine.
Vaccines and Herd Immunity
“Herd immunity occurs when a large portion of a community – the herd – becomes immune to a disease, making the spread of the disease from person to person unlikely. As a result, the whole community becomes protected – not just those who are immune”
Mayo Clinic
There are two paths that lead to herd immunity – vaccinations and natural infection. The latter is not a reliable pathway towards herd immunity against COVID-19 because it isn’t yet clear if a COVID-19 infection provides substantial, long-term immunity. Additionally, an estimated 70% of the population – 46.2 million people in the UK – would need to be infected to provide herd immunity to the remainder. This will lead to a host of other problems, from a possible collapse of healthcare infrastructure to a large number of deaths.
Consequently, vaccines provide the ideal opportunity to achieve herd immunity against the virus. Herd immunity following mass inoculations is an approach that has proved extremely successful in the past. Various deadly contagious infections such as smallpox, polio and rubella have been contained due to national vaccination programs. These are important examples to think about when considering the efficacy of a mass vaccination campaign.
Ultimately, large numbers of the public will need to be vaccinated for herd immunity to be achieved. It is worth reflecting on the barriers to this in today’s climate. Some of these include growing ‘anti-vax’ sentiments, lack of trust in the healthcare system amongst BAME communities as well as the logistics of attaining and distributing the vaccines.
Kickstart your Interview Prep
UK COVID-19 vaccine rollout
The NHS is currently only offering the vaccine to those most at risk of infection. This includes people aged 80+ years, those who are clinically vulnerable, care home residents and workers, health and social workers in England. Prioritization may look slightly different in Scotland, Wales and Northern Ireland. The UK government has stated that doctors and all healthcare professionals working in England will need to be fully vaccinated from 1 April 2022 unless exempt.
As of 31st December 2021, 33.5 million booster jabs have been administered in the UK following the booster programme which began in September 2021. You can keep up to date on this information using the following resources:
Top Tip: Read up on the latest vaccine roll-out guidance at the places where your university is located prior to attending the interview.
According to the NHS website, the vaccine is not currently available for children under the age of 12. As for pregnant and/or breastfeeding women, there is no evidence to suggest that the vaccine is unsafe but more research is required. As a result, the vaccine is only given to those who are pregnant and/or breastfeeding if they work in a high-risk environment or have co-morbidities that put them at risk of a severe COVID-19 infection.
What to expect in your interviews
With so much information available at your fingertips and a whole host of possible interview questions, let’s delve into what to expect in your interviews. To begin with, broadly reflect on the purpose of medical school interviews. Universities want to learn more about their candidates; they want to know who you are and what you can offer to their institution and the NHS.
Keeping this in mind, questions surrounding COVID-19 will most likely focus on ethical conundrums and reflections. Some of the common questions to prepare for include:
- How has COVID-19 changed your perception of medicine/affected your motivation to pursue a career in medicine?
- What do you know about the COVID-19 vaccines?
- What do you understand about the testing and regulatory processes of vaccine production, focussing on the COVID-19 vaccines?
- Should the COVID-19 vaccine be made compulsory?
- Should the public have to pay for the COVID-19 vaccine?
- Who should receive the vaccine first? Other variations of this question include whether or not particular groups, the elderly, for example, should be given the vaccine first.
- What can be done to increase the uptake of the COVID-19 vaccine?
- How is social media affecting the uptake of the COVID-19 vaccine?
How to answer the most anticipated vaccine-related questions
For questions surrounding the impact of COVID-19 on your motivation to apply to medical school, focus on personal reflections and learning objectives. Ask yourself the following questions:
- How has COVID-19 changed the healthcare environment?
- Has COVID-19 changed your perception of public health, research etc?
- What have you learnt about the roles of doctors during the pandemic?
- Have you done any work experience in the pandemic and if so, what did you learn?
Top Tip: Remember to avoid extremes in your answer – for example, don’t suggest that the pandemic hasn’t affected your motivation at all but don’t communicate a completely negative perspective either.
When it comes to ethical questions, think about the 4 pillars of medical ethics – beneficence, non-maleficence, justice and autonomy. Additionally, consider ethical adjuncts such as patient safety, consent, confidentiality etc. The key to ethical stations is to strive for a balanced answer. Delve into the advantages and disadvantages of the proposal in question before verbalizing your ultimate opinion. This shows critical and analytical thinking as well as the ability to remain unbiased and rational in difficult situations.
As a general rule of thumb when preparing for pandemic-related MMI scenarios, read up on the latest news prior to your interviews. BBC Health, BMJ and other similar sources are good places to start. Remember to fact-check the information you obtain from media sources and weigh up both sides of every argument. Finally, brush up on your knowledge of vaccines and acquired immunity.
The MMI is a unique interview experience like no other; it tests passion, motivation, dedication and proactiveness. COVID-19 drew the curtains on various government and healthcare systems around the world. It exposed strengths, flaws and urgent areas for improvement moving forward. There is no doubt that medical school interviewers will reflect on this; after all, you are the future of the NHS. Therefore, questions about the pandemic are to be expected. This guide is only the first step to the research and reflection that you must put in to show your interviewers that you are the perfect candidate for their medical school. Happy reading & good luck!

Frequently Asked Question
→What are COVID-19 vaccines, and how do they work?
COVID-19 vaccines are vaccines that protect against the coronavirus disease. They work by triggering an immune response in the body that helps prevent infection or reduce the severity of the disease if infected.
→Are COVID-19 vaccines safe?
Yes, COVID-19 vaccines are safe and have undergone rigorous testing and clinical trials to ensure their safety and efficacy. Millions of people have received COVID-19 vaccines with few reported side effects.
→How many types of COVID-19 vaccines are there, and what are their differences?
There are currently several types of COVID-19 vaccines, including mRNA vaccines, viral vector vaccines, and protein subunit vaccines. Each type works differently, but they all have been shown to be safe and effective in preventing COVID-19.
→What are the side effects of COVID-19 vaccines?
Common side effects of COVID-19 vaccines include pain or swelling at the injection site, fever, headache, fatigue, and muscle aches. These side effects are usually mild and go away on their own within a few days.
→How effective are COVID-19 vaccines?
COVID-19 vaccines have been shown to be highly effective in preventing COVID-19 infection and reducing the severity of the disease if infected. The effectiveness of the vaccines may vary depending on the type of vaccine and the individual’s age and health status.
→Can you still get COVID-19 after getting vaccinated?
It is possible to still get COVID-19 after getting vaccinated, but the vaccines have been shown to significantly reduce the risk of infection and severe illness. If you do get infected after vaccination, you are less likely to have severe symptoms or require hospitalization.
→Should everyone get vaccinated against COVID-19?
Yes, everyone who is eligible should get vaccinated against COVID-19 to protect themselves and others from the disease. Vaccination is an important tool in controlling the spread of COVID-19 and ending the pandemic.
→What is the importance of COVID vaccination?
The COVID-19 vaccination is important for several reasons:
Protection against COVID-19: The most obvious benefit of COVID vaccination is that it protects you from getting COVID-19 or reduces the severity of the illness if you do get infected.
Protection of others: By getting vaccinated, you are not only protecting yourself but also those around you who may be at higher risk of severe illness or cannot get vaccinated due to medical reasons.
Controlling the spread of COVID-19: Vaccination is an important tool in controlling the spread of COVID-19 and ending the pandemic. The more people who get vaccinated, the less the virus can spread, which can help to reduce the number of cases, hospitalizations, and deaths.
Preventing mutations: When the virus spreads rapidly, it has more opportunities to mutate and potentially create new variants that may be more contagious or resistant to existing vaccines. Vaccination can help to reduce the spread of the virus, which in turn can reduce the risk of new variants emerging.
Return to normalcy: Vaccination can help us return to some sense of normalcy by allowing us to gather in larger groups, travel, and engage in other activities that have been restricted due to the pandemic.
In summary, COVID vaccination is important not only for personal protection but also for the protection of others, controlling the spread of the virus, preventing mutations, and returning to a more normal way of life.
Related Articles
Related links
Get instant feedback on responses to questions to hone your answers.
Gain access to 100+ tutorials, practice stations and online content including university-specific information.
20 station circuit where you'll gain insight and practice for your medical interview.





Was this article helpful?
Still got a question? Leave a comment
Leave a comment